This case comes from Sam Ghali (@EM_RESUS).
A 60-year-old man calls 911 after experiencing sudden onset chest pain, palpitations, and shortness of breath. Here are his vital signs:
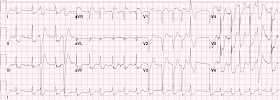
Here is the repeat ECG obtained 25 minutes after the first one:
A 60-year-old man calls 911 after experiencing sudden onset chest pain, palpitations, and shortness of breath. Here are his vital signs:
HR: 130-160, BP: 140/75, RR:22, Temp: 98.5 F, SaO2: 98%
This is his 12-Lead ECG:
ECG reading is all about pattern recognition. And this particular pattern of ST-Elevation in aVR with diffuse ST Depression is a very important ECG pattern that you must be able to recognize. But what's probably more important than being able to recognize the pattern, is understanding what it represents. There appears to be a common misconception that the ST-Elevation in aVR in this case possibly represents "STEMI", or acute transmural (full-thickness) ischemia. If this were the case the patient would most likely be dead or at the very least in profound cardiogenic shock. The key to understanding what this pattern represents lies in understanding that the ST-Elevation in aVR is reciprocal to the diffuse ST-Depression - and that this diffuse ST-Depression represents global subendocardial ischemia!
So the real question that you must answer is:
What is causing the global subendocardial ischemia?
It is critical to realize that more often than not the cause is global myocardial strain from a Non-ACS etiology!
(profound sepsis, tachycardia, anemia, hypoxemia, etc). It is also very
important to understand that in these Non-ACS settings, you can see
this pattern with or without underlying coronary artery disease.
But
of course it could be ACS. And if it is, then you are dealing with Left
Main, Proximal LAD, or even multi-vessel plaque instability. But keep
in mind that even if it is ACS you are still dealing subendocardial and
not transmural ischemia.
Here is a subcostal view of the bedside Echo obtained from our patient in the ED:
 |
| There is good global function |
So
what is causing the diffuse subendocardial ischemia in our patient?
When the heart rate is significantly elevated as in this case, it is
reasonable to suspect that the ischemia is likely tachycardia-induced,
or "demand ischemia." So given the normal EF noted on Echo, (and by the
way I would strongly recommend assessing the EF of any patient before
deciding to give any negative inotropic medications) the decision was
made to administer a Diltiazem bolus and infusion and to reassess after
rate control was established. Rate
controlled was gained as the patient's heart rate came down very nicely
into the 80's. He felt much better and his symptoms were all but
completely relieved.
Smith comment: it is also critical to assess volume before giving negative inotropes and negative chronotropes. This tachycardia could be a response to poor LV filling. Indeed the neither LV nor RV appear to be filling very well. If the atrial fib with RVR is resulting in a rate so fast that the rate is the cause of poor LV filling, then there should be some increased filling pressures, possible pulmonary edema, and evidence of fluid overload. Assessment of IVC filling would be helpful, and, if it is collapsed, then administration of fluids first (or blood if this is a GI bleed) is indicated. If this does not result in a slower heart rate, then an AV nodal blocker is indicated, such as Diltiazem.
Furthermore, since this patient has no history of atrial fib, and it is a critical situation, electrical cardioversion is both safer and more effective than an AV nodal blocker such as Diltiazem.
Furthermore, since this patient has no history of atrial fib, and it is a critical situation, electrical cardioversion is both safer and more effective than an AV nodal blocker such as Diltiazem.
See these 2 posts:
Atrial fibrillation with RVR: use POCUS to assess volume; then sinus vs. SVT: use of Lewis leads
Here is the repeat ECG obtained 25 minutes after the first one:
If
this repeat ECG had shown resolution of the global subendocardial
ischemia pattern, it would be reasonable to conclude that it was likely
the result of a-fib with an uncontrolled ventricular response. But
because this pattern persisted after rate control and in the absence of
any other evidence of clinical causes, one must assume that the etiology
of the pattern is indeed ACS - meaning there has been acute plaque
instability in either the left main coronary artery, Proximal LAD, or
multi-vessel involvement.
The
patient was started on heparin in addition to the aspirin he received
en route and the Cardiology team was consulted. (Of note, it is
important not to start these patients
on dual anti-platelet therapy as there is a high likelihood that they
will require CABG.) The decision was made to proceed urgently to the
cath lab for angiography.
Cardiac Cath Results:
Left Main: There is a 90-95% stenosis of the distal left main including the ostium of LAD and Left Circumflex arteries.
LAD: There is a focal 80% stenosis just after the takeoff of the first diagonal branch.
Circumflex: Severe disease at its ostium and moderate disease in the remainder of the proximal segment.
RCA: 100% chronic total occlusion at its proximal segment.
In
discussion with the interventional cardiologist who performed the cath
there was thought to be evidence of likely a component of acute thrombus
at the 90-95% left main stenosis, suggesting Left Main ACS!
Case Resolution:
The patient was referred for CABG and ended up doing quite well.
Take Home Points:
1. The key to ECG reading is pattern recognition. The
pattern of ST-Elevation of at least 1mm in lead aVR + diffuse
ST-Depression with a maximal depression vector towards leads II & V5
is a pattern you must know. It represents global subendocardial ischemia
2. When you see this pattern you should divide the differential for the diffuse subendocardial ischemia into two main categories: ACS vs Non-ACS. Do not automatically assume that it is ACS. I
have seen this mistake made many times as ACS becomes the focus, at the
expense of appropriate resuscitation addressing the underlying cause. It is very important to keep in mind that the etiology is far more likely to be Non-ACS than ACS!
See this case:
Diffuse Subendocardial Ischemia on the ECG. Left main? 3-vessel disease? No!
3.
The key to determining the etiology is through history, physical exam,
clinical picture, laboratory data, Echo, and vigilant monitoring and
frequent reassessment. If you have identified and addressed potentially
reversible causes of the ischemia, and the ECG pattern persists then
you are dealing with ACS until proven otherwise.
4. Refrain from using dual-antiplatelet therapy in these patients as there is a high likelihood they will require CABG.
5. Remember that if this ECG pattern does represent ACS, the ST-Elevation in aVR is not the result of direct injury (or transmural ischemia) and that the ST-Elevation in aVR is reciprocal to the diffuse ST-Depression. Therefore these ACS cases do not represent
"STEMI". However, while there is not great data to guide the timing of
cath for these patients, I would advocate going to the cath lab with a
much stronger sense of urgency than for other "NSTEMIs". The reasoning
is that ACS is a very dynamic process and without the advantage of
optimal medical therapy (a second platelet inhibitor should be withheld)
there is a higher chance of the culprit vessel suddenly occluding and
evolving to transmural ischemia. If this happens in the Proximal LAD,
Left Main, or in the setting of Multi-vessel involvement the myocardial
territory in jeopardy is so large that there is a good chance the
patient will arrest and die before any reperfusion can be established!
6. Smith comment: With diffuse subendocardial ischemia, you may not see any wall motion abnormality. Global function can even be normal, although it may be globally depressed as well. A normal bedside echo does not help in: 1) differentiating the cause of the STE in aVR 2) ruling out ACS.
See this previous post:
Here is the section on aVR written by Smith in: Miranda et al. "New Insights into the Use of the 12-lead ECG in Acute MI in the ED" (Canadian J Cardiol 34(2):132-145; Feb 2018)
Lead aVR in ACS 62
Many experts consider the ECG pattern of STE in aVR, with diffuse STD elsewhere (referred to herein as the “aVR STE pattern”), to be representative of LM ATO.7 The 2013 ACC/AHA STEMI guidelines consider this a “STEMI equivalent,” in which thrombolytic therapy is not contraindicated (evidence level B, no specific class of recommendation). 18 However, these conclusions are on the basis of studies in which LM lesions were not true subtotal or complete occlusion (ie, TIMI 0/1 flow).62,63 The interventional community defines occlusive LM disease as >50% according to fractional flow reserve, or 75% stenosis,64 but urgent or emergent intervention on lesions not meeting these thresholds is only imperative if it is a thrombotic lesion and the patient has refractory ischemic symptoms (ie, not resolved by nitrates, antiplatelet, and antithrombotic therapies; see 3 examples in Supplemental Fig. S7).
Although nearly half of patients with 1 mm STE in aVR due to ACS will require coronary artery bypass surgery for revascularization,62 the infarct artery is often not the LM, but rather the LAD or severe 3-vessel disease. More importantly, such ECG findings are frequently due to nonocclusive etiologies (eg, baseline LVH, demand ischemia secondary to respiratory failure, aortic stenosis, hemorrhagic shock). Knotts et al. reported that only 23% of patients with the aVR STE pattern had any LM disease (fewer if defined as 50% stenosis). Only 28% of patients had ACS of any vessel, and, of those patients, the LM was the culprit in just 49% (14% of all cases).57 It was a baseline finding in 62% of patients, usually due to LVH.
Thus, a number of expert reviews emphasize the low specificity of the aVR STE pattern, preferring to label it as circumferential subendocardial ischemia; in this syndrome, STE in aVR is reciprocal STE, reciprocal to an STD vector toward leads II and V5.10,12,62
The aVR STE pattern is also not sensitive for LM ATO. However, anterior STEMI with combined new right bundle branch block and left anterior fascicular block is highly suggestive of LM ATO (see example 12-lead ECG in Supplemental Fig. S8).65,66
It should be re-emphasized that true LM ATO (ie, TIMI flow 0) is rare in the ED, because most either die before arrival or are recognized clinically because of cardiogenic shock. Thus, reported specificities of STE in aVR for LM ATO result in very low positive predictive values. Of those who do get to the ED, many present with clear STE.62,65,66
The ACC/AHA states that thrombolytics are not contraindicated for diffuse STD “ associated with” STE in aVR. Because of the poor specificity of this pattern for LM ATO, we suggest that thrombolytics should only be considered for those with profound STD that is clearly due to ACS, is refractory to all other medical management, and only when PCI is completely unavailable.
Lead aVR in STEMI
Some patients whose ECGs already meet conventional STEMI criteria might also have STE in lead aVR. This finding does not alter the need to pursue emergent reperfusion, although it might suggest a poorer prognosis.62,67 In a patient with otherwise diagnostic STE, additional STE in aVR does not represent LM ATO and is not helpful in diagnosing the infarct-related artery or the site of occlusion.68 Less than 3% of anterior STEMI has LM ATO, and most are recognized clinically because of cardiogenic shock.69,70
References
62. Smith SW. Updates on the electrocardiogram in acute coronary syndromes. Curr Emerg Hosp Med Rep 2013;1:43-52.
63. Jong GP, Ma T, Chou P, et al. Reciprocal changes in 12-lead electrocardiography
can predict left main coronary artery lesion in patients with acute myocardial infarction. Int Heart J 2006;47:13-20.
64. Stone GW, Sabik JF, Serruys PW, et al. Everolimus-eluting stents or bypass surgery for left main coronary artery disease. N Engl J Med 2016;375:2223-35.
65. Fiol M, Carrillo A, Rodriguez A, et al. Electrocardiographic changes of ST-elevation myocardial infarction in patients with complete occlusion of the left main trunk without collateral circulation: differential diagnosis and clinical considerations. J Electrocardiol 2012;45:487-90.
66. Widimsky P, Rohac F, Stasek J, et al. Primary angioplasty in acute myocardial infarction with right bundle branch block: should new onset right bundle branch block be added to future guidelines as an indication for reperfusion therapy? Eur Heart J 2012;33:86-95.
67. Kukla P, Bryniarski L, Dudek D, Krolikowski T, Kawecka Jaszcz K. Prognostic significance of ST segment changes in lead aVR in patients with acute inferior myocardial infarction with ST segment elevation. Kardiol Pol 2012;70:111-8.
68. Kosuge M, Ebina T, Hibi K, et al. An early and simple predictor of severe left main and/or three-vessel disease in patients with non-ST-segment elevation acute coronary syndrome. Am J Cardiol 2011;107:495-500.
69. Zoghbi GJ, Misra VK, Brott BC, et al. ST elevation myocardial infarction due to left main culprit lesions: percutaneous coronary intervention outcomes. J Am Coll Cardiol 2010;55:A183.E1712.
70. Kurisu S, Inoue I, Kawagoe T, et al. Electrocardiographic features in patients with acute myocardial infarction associated with left main coronary artery occlusion. Heart 2004;90:1059-60.


My comments added to this great blog post by Dr. Stephen Smith commenting on the case by Sam Ghali @EM_RESUS):
ReplyDeleteThis case is the perfect discussion surrounding ST-Elevation in aVR and how it is a reciprocal to diffuse ST-Depression (as opposed to transmural STEMI), almost in the ‘opposite’ vector of Lead II/V5. It is representative of Global Subendocardial Ischaemia; the main question always being ‘What is causing it?’.
I really like the section surrounding the NON-ACS aetiology, especially considering it is just as likely (probably more likely) than ACS (for example sepsis, tachycardia, anaemia, hypoxaemia) for a wider consideration of working diagnosis; but also the importance that if we are dealing with LMCA, Proximal LAD or 3VD (plaque instability), that we are still presented with subendocardial ischaemia on ECG and not a diagnostic transmural infarction (this patient would most likely present in, or certainly extremely near, cardiac arrest (or severe cardiogenic shock!).
However, following clinical tests/examination and rate control with Diltiazem (AV blocking ability), the aVR STE persists and we are left with severely poor cardiac cath results suggesting Left Main ACS.
Great points quoted:
- If you have identified and addressed potentially reversible causes of the ischaemia, and the ECG pattern persists, then you are dealing with ACS until proven otherwise.
- With diffuse subendocardial ischaemia, you may not see any wall motion abnormalities on echo.
- A normal bedside echo does not rule out ACS!
Great review on the topic of STE in aVR combined with STD in several leads!
ReplyDeleteI'd add that the European guidelines (2018) seem more prudent (though more 'diplomatic' as well) as to reperfusion therapy: they recommend prompt percutanous coronary intervention (of course in an appropriate clinical context) and do not mention at all thrombolytics, perhaps given that many patients will require surgical coronary revascularization, thus sparing them lytic risks (and on the other hand with no benefits).
Thank you.... Great explanation as always.
ReplyDeleteIn case of STE aVR with ST depression in all other leads is it mandatory to calculate GRACE 2.0 or TIMI score or the patient needs to go for Cath immediately? What's done in practise?
ReplyDeleteI'm not sure that's terribly helpful. If the ECG findings are indeed due to ACS, and you cannot get the pain and the ST depression (and reciprocal STE in aVR) to resolve with medical therapy (fluids, nitro, etc.), then emergent cath lab is indicated. Even if they do resolved (if it is ACS), the sooner the cath, the better.
DeleteIn the case of resolution would that be classified as "unstable angina"?
DeleteI have recently come across a case of a middle-aged gentleman presenting with chest pain. He gave a history of recurrent exertional chest pain in the past but had not seek medical attention previously. ECG on admission showed obvious STE in aVR (nearly 2mm) and significant diffuse ST depression. He was given sublingual nitrates; his chest pain improved and repeat ECG in 30 minutes showed near complete resolution of almost all ST changes.
He had recurrent chest pain within the next 30 minutes and repeat ECG again showed the diffuse ischemic changes, which was resolved by a second dose of sublingual nitrates. This scenario was repeated again the next hour.
How would you gauge the need for cath lab in this case? Also would you tend to withhold Plavix for such cases?
Thanks!
There is no correct answer, but I would give it only one chance to resolve. If there is recurrent ischemia, go to the cath lab!
DeleteA very insightful case and great explanation as always.. Thanks for representing the case.
ReplyDeleteThanks!
DeleteThis comment has been removed by the author.
ReplyDeleteExcellent post by Drs. Sam Ghali and Stephen Smith on the importance of recognizing the pattern of diffuse ST depression in association with ST elevation in lead aVR. I’d add the following points: i) It is the remote superior and right-sided electrical viewpoint of lead aVR (looking down at the heart from the right shoulder) that provides a unique vantage point that assesses the basal part of the interventricular septum. Ischemia of the septum (as may occur with severe LMain disease) produces a vector that points superiorly — which results in ST elevation in lead aVR. ii) Diffuse subendocardial ischemia typically also produces ST elevation in lead aVR — here, as a reciprocal change to the diffuse ST depression that is seen in most other leads on the tracing. iii) A clue for distinction between LMain vs proximal LAD disease may be suggested by the relative amount of ST elevation in lead V1 compared to aVR (LMain disease being more likely to produce ST elevation in lead aVR > V1 — vs proximal LAD involvement being more likely to produce relatively more ST elevation in V1). iv) Various patterns of severe multi-vessel coronary disease may produce various permutations of this theme — so that distinction between LMain vs proximal LAD or multi-vessel disease, or severe non-coronary factors — OR some combination of all of these — will not always be possible from the initial ECG. That said, it is not surprising in this case that the profound diffuse ST depression seen on the initial ECG was associated with severe multi-vessel coronary disease. Nor is it surprising given >3mm of ST elevation in aVR (considerably more than the ST elevation in lead V1) that there was also LMain involvement. THANKS to Drs. Ghali and Smith for presenting this insightful case!
ReplyDeleteGreat case illustrating the importance of ecg interpretation.Regarding the treatment ,would you not like to use iv amiodarone rather than diltiazem to increase changes of reversion to sinus rythm?
ReplyDeleteI would probably have electrically cardioverted.
DeleteGreat case and wonderful explanation as usual! Congrats!If there was no history of AF and the patient presented with Af with RVR and the ischemic ECG pattern, would it be a reasonable choice to proceed to immediate cardioversion?How would this affect this patient?
ReplyDeleteYes. I should have put this in! Electrical Cardioversion would be best
DeleteRefrain from using dual-antiplatelet therapy in these patients as there is a high likelihood they will require CABG.
ReplyDeleteCan you explain why please?
In ACS (and there are other reasons for this ECG pattern!), this pattern suggests Left Main or 3 vessel disease and the patient has 50% likelihood of needing CABG. P2Y12 inhibitors make surgery very risky.
Delete